DCTs or “virtual” clinical trials will continue to grow in utilization offerings within the pharmaceutical industry. In order to meet this opportunity, pharma companies must holistically view their DCT strategy. DCTs are an innovative way to achieve enhanced patient centric clinical trials.
Traditional Clinical Trials in the Digital Age
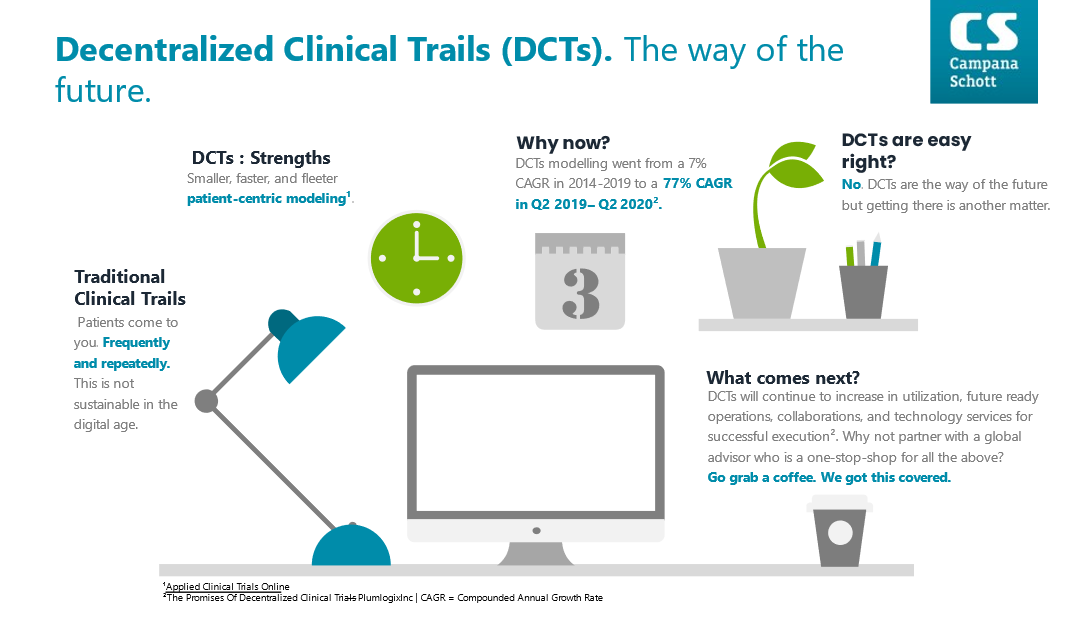
Traditional clinical trials are a pillar of modern medicine, but they are not free of critical short comings. Central to traditional clinical trials is the need for patients to come to you in some capacity. In specific, traditional clinical trials typically centralize their storage and operational centers which can dramatically impact patient adherence, access, and inclusivity.
Paired with the challenges of recruitment and retention, traditional clinical trials are increasingly out of touch with modern times and digital technology.
Will traditional clinical trials disappear soon? Unlikely. Is a noted shift occurring towards the increased utilization of DCTs? The short answer is a resounding yes.
DCTs: Overview
DCTs strengths can be summarized into three simple concepts: smaller, faster, and nimbler. DCTs are smaller and can be targeted to reach under-served populations which would traditionally face significant challenges to clinical trial participation. DCTs are faster due to the ability to scale quickly relative to participant enrollment, smart tools as enablers for data monitoring, paired with the limited need to physically attend a trial center. DCTs are nimbler in that they can be modified rather easily due to the strong use of digital technology to collect, aggregate, and disseminate data to the correct parties.
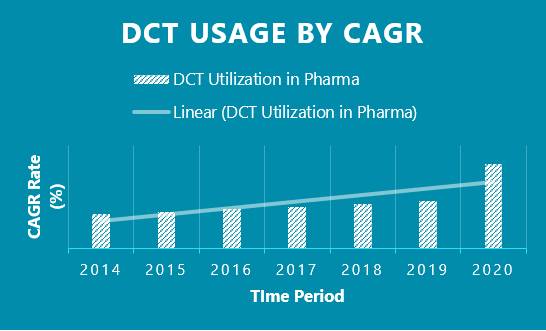
DCTs have seen a 10-fold increase in utilization from 2014 -2019 vs. 2019-Q2 2020. Covid-19 has been the main driving force behind the explosion in popularity for DCTs. For the first time, DCTs were truly seen as a viable option to clinical trials due to the forced realities that Covid-19 brought to the global community.
DCTs: Weakness
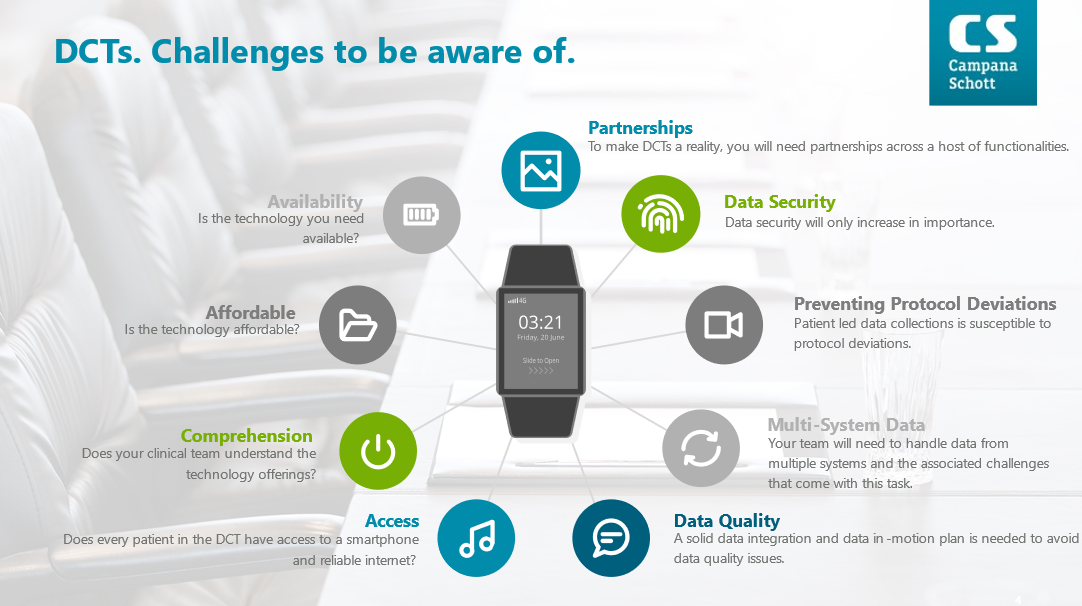
DCTs have several advantages as denoted above but they are not a magical bullet either. DCTs require extensive levels of coordination to avoid patient burn-out (e.g., redundant applications, at home data collection challenges, behavioral incentives, etc.). DCTs face shifting regulatory modalities (e.g., telemedicine, direct-to-patient product shipments, etc.).
DCTS also require a new skillset relative to design, operations, and execution of the trial itself. Gone is the ability to house all your resources under “one-roof”. Rather for a DCT to be successful you need a strong master data management (MDM) strategy, industry leading data security mechanisms, sophisticated data analytics, solid distribution operations, and hands on partnerships with experienced global project management firms to handle the hundreds of intricacies which arise from at-home clinical trials.
Managing the risks and opportunities of DCTs will be the principal driver for the pharma industry. Managing the risks and opportunities of DCTs will be the principal driver for the pharma industry.
DCTs: What comes next?
DCTs will continue to increase in utilization, future ready operations, collaborations, and technology services. DCTs are well accepted by patients, showing up to a 20% increase in patient-adherence against traditional models. Up to 80% of medical studies are non-reproducible due to data collection errors and interpretation misalignments. The possibility to mitigate this reproducibility crisis may soon be achievable via DCTs and blockchain. Any pharma firm making operational and or strategic shifts towards DCTs must take a holistic approach to this surging trial design methodology. Managing the risks and opportunities of DCTS will be the principal driver for the pharma industry in the near-to-medium term. With 30 years of global industry experience Campana & Schott is ready to support you on your journey to successful DCT implementation. Whether you are in the ideation, pre-clinical, clinical, or simply looking for a sparring partner phase, Campana & Schott is here for you. Go grab a coffee, we got you covered.

